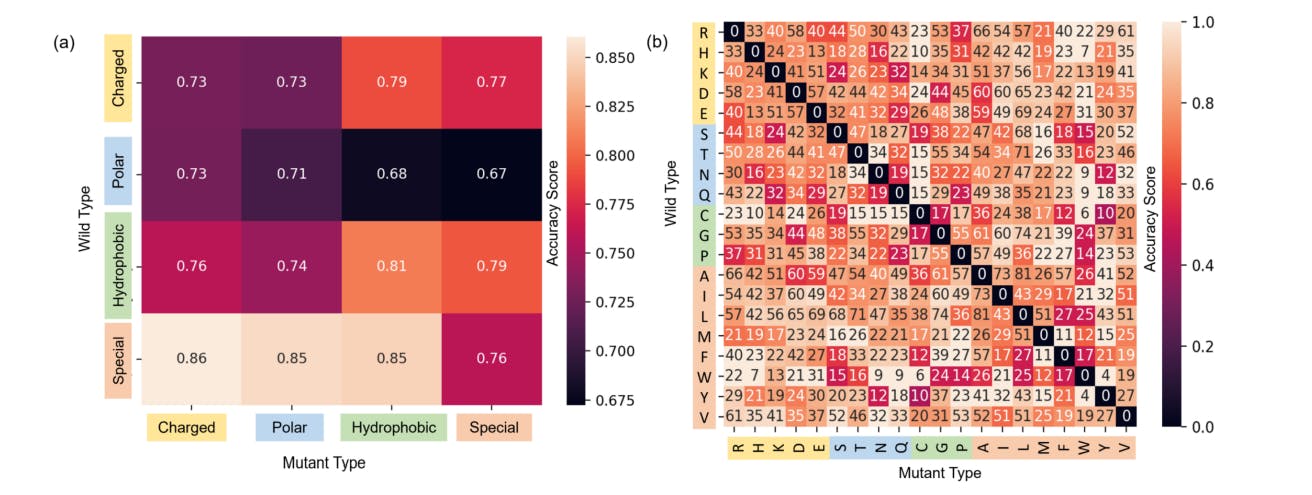
110 reads
TopLapGBT: Bridging Gaps in Protein Solubility Prediction with Cutting-Edge Fusion
by
February 17th, 2024
Audio Presented by
Mutation: process of changing in form or nature. We publish the best academic journals & first hand accounts of Mutation
About Author
Mutation: process of changing in form or nature. We publish the best academic journals & first hand accounts of Mutation